Why don’t Covid vaccines target membrane (M) protein to resist immune escape and induce neutralizing immunity beyond that from spike protein?
Authorized vaccine makers won’t say why their jabs don’t utilize the virus’s immunogenic, mutationally stable, and apparently nontoxic M protein (Updated 7/18/22)
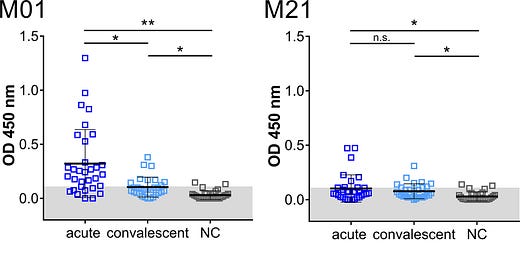
Figure 2: IgM immuno-reactivity obtained with immunodominant peptides in the acute and convalescent phase and negative control group (NC). Each data point represents the OD450 value of one patient. Mean and SD are indicated. The threshold level (mean + 2SD of control) is marked in gray. Data shown is representative of five independent experiments. Asterisk indicates significance *P < 0.05, **P < 0.0001. n.s., not significant. M: membrane protein. S: spike protein. N: nucleocapsid.
From Jörrißen et al. (2021) Antibody Response to SARS-CoV-2 Membrane Protein in Patients of the Acute and Convalescent Phase of COVID-19.
Key points:
Authorized Covid vaccines solely target the toxic, full-length SARS-CoV-2 (SARS-2) spike (S) protein, and no approved vaccine maker has chosen also to target the SARS-2 membrane (M) protein, suggested in studies to be nontoxic and immunogenic, producing T cell responses and high antibody titers in Covid patients.
Because antibodies to M protein of the SARS-CoV (SARS-1) virus — nearly identical to the SARS-2 M protein — have been shown to neutralize the virus, researchers expect antibodies to the SARS-2 M protein also to be neutralizing, though this needs further confirmation.
Covid vaccines targeting only the rapidly evolving spike protein — whose mutation rate was enhanced at least 20 fold through exonuclease alterations identical to ones engineered from SARS-1, leading to evolution of some Omicron variants — don’t significantly block viral transmission and are designed for selection of more infectious and lethal variants (see also here and here).
Vaccine targeting of the M protein is expected to reduce vaccinal immune escape by variants and improve virus neutralization, helping to achieve herd immunity.
Because rapid immune escape from spike protein vaccines by viral variants — preventing herd immunity — is almost certainly an intended vaccine design feature and not merely incidental to the Covid bioweapon operation, this could explain why vaccine makers don’t use, or even discuss using, the M protein in Covid vaccines.
Below are Covid M protein perspectives.
Geert skirts M
From the video discussion above between Bret Weinstein and virologist Dr. Geert Vanden Bossche:
29:55: Bret Weinstein suggests that including more antigens than the spike protein in Covid vaccines would be useful for reducing immune escape.
31:10: Geert Vanden Bossche replies that the spike protein is “the right target” as a vaccine antigen because it “enables infection into the cell” and that the nucleocapsid (N) protein would not be a good target as it is inaccessible to circulating antibodies — valid points supported by the literature.
Surprisingly, Vanden Bossche doesn’t mention M protein as an additional candidate vaccine antigen that is accessible to circulating antibodies and produces strong IgM and IgG responses in nearly all Covid patients and, at least in SARS patients, “efficient neutralizing antibodies,” according to Liu et al.
Lopandic et al. (2021): IgM and IgG Immunoreactivity of SARS-CoV-2 Recombinant M Protein
In ELISA, more than 93% (28/30) of COVID-19 sera were positive for IgM detection, and more than 96% (29/30) were positive for specific IgG detection to M protein.
Successful design and computer modeling of an M-protein Covid vaccine
Ayyagari and colleagues designed a membrane (M) protein Covid vaccine that elicited successful immune stimulation responses in silico, meaning in a computer model.
Immune simulation studies showed the induction of primary, secondary and tertiary immune responses marked by the increased levels of antibodies, INF-γ, IL-2, TGF-β, B-cells, CD4+ and CD8+ cells.
Finally, the vaccine construct was able to elicit immune response as desired.
Ayyagari et al. (2020): Design of a multi-epitope-based vaccine targeting M-protein of SARS-CoV2: an immunoinformatics approach
M protein antibodies are expected to be neutralizing
Lu et al. (2021): The immunodominant and neutralization linear epitopes for SARS-CoV-2
M183–197 is another immunodominant epitope in domestic cases. Because this epitope is located in the S4 subsite of the active center of the M protein, it is possible for it to elicit neutralizing antibodies that inhibit the protease function of the M protein (Dai et al., 2020; Yang et al., 2005).
M protein has strong antigenic properties and is involved in viral entry into host cells
Lopandic et al. (2021): IgM and IgG Immunoreactivity of SARS-CoV-2 Recombinant M Protein
Abstract:
Diagnostic evaluation of specific antibodies against the SARS-CoV-2 virus is mainly based on spike (S) and nucleocapsid (N) proteins.
Despite the critical functions in virus infection and contribution to the pattern of immunodominance in COVID-19, exploitation of the most abundant membrane (M) protein in the SARS-CoV-2 serology tests is minimal.
This study investigated the recombinant M protein’s immunoreactivity with the sera from COVID-19 convalescents. In silico designed protein was created from the outer N-terminal part (19 aa) and internal C-terminal tail (101–222 aa) of the M protein (YP_009724393.1) and was recombinantly produced and purified. ?
The designed M protein (16,498.74 Da, pI 8.79) revealed both IgM and IgG reactivity with serum samples from COVID-19 convalescents in Western blot. In ELISA, more than 93% (28/30) of COVID-19 sera were positive for IgM detection, and more than 96% (29/30) were positive for specific IgG detection to M protein.
Based on the capacity to provoke an immune response and its strong antigenic properties, as shown here, and the fact that it is also involved in the virion entry into host cells, the M protein of the SARS-CoV-2 virus as a good antigen has the potential in diagnostic purposes and vaccine design.
SARS-2 was likely engineered to increase spike protein’s mutation rate and immune escape from vaccines
The SARS-2 spike protein appears to have been engineered to mutate at least 20 times faster than usual through changes to an exonuclease in SARS-1, leading to evolution of some vaccine-resistant Omicron variants.
Therefore, any vaccine expected to reduce escape by the virus from vaccinal immunity, thereby reducing transmission in an effort leading to herd immunity, should consider additional use of other, more stable antigens such as M protein.
M protein regions are mutationally stable and might resist vaccinal immune escape
Jörrißen et al. (2021) Antibody Response to SARS-CoV-2 Membrane Protein in Patients of the Acute and Convalescent Phase of COVID-19:
As peptides M01 and M21 are located in two highly conserved regions of the SARS-CoV-2 genome, such antibodies may evade the higher variations observed for other epitopes of SARS-CoV-2 structural proteins.
CD8+ T cell responses after vaccination of monkeys with only SARS-CoV-2 N, M, and E antigens — no spike (S) — correlated with reduced nasopharyngeal viral load
Ishii et al. (2022): Neutralizing-antibody-independent SARS-CoV-2 control correlated with intranasal-vaccine-induced CD8+T cell responses
Effective vaccines are essential for the control of the coronavirus disease 2019 (COVID-19) pandemic.
Currently developed vaccines inducing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S)-antigen-specific neutralizing antibodies (NAbs) are effective, but the appearance of NAb-resistant S variant viruses is of great concern.
A vaccine inducing S-independent or NAb-independent SARS-CoV-2 control may contribute to containment of these variants.
Here, we investigate the efficacy of an intranasal vaccine expressing viral non-S antigens [using plasmid DNAs expressing nucleocapsid (N), membrane (M), and envelope (E) antigens] against intranasal SARS-CoV-2 challenge in cynomolgus macaques.
Seven vaccinated macaques exhibit significantly reduced viral load in nasopharyngeal swabs on day 2 post-challenge compared with nine unvaccinated controls.
The viral control in the absence of SARS-CoV-2-specific NAbs is significantly correlated with vaccine-induced, viral-antigen-specific CD8+ T cell responses.
Our results indicate that CD8+ T cell induction by intranasal vaccination can result in NAb-independent control of SARS-CoV-2 infection, highlighting a potential of vaccine-induced CD8+ T cell responses to contribute to COVID-19 containment.
M protein in SARS-1, nearly identical to that in SARS-2, elicits neutralizing antibodies
Of the closely related SARS-1 virus:
The antigenicity of specific proteins within a viral proteome differs depending on the diversity of quantity and immunogenic antigenicity of the immune epitopes comprised in the proteins.
It has been demonstrated that structural proteins of SARS-CoV such as spike (S), nucleocapsid (N), membrane (M), and envelope (E) proteins possess much higher immunogenicity for T cell responses than the nonstructural proteins.
It has been shown that the S and N proteins are characterized by high immunogenicity to engage the humoral and cellular response to SARS-CoV.
The M and E proteins are the other 2 significant structural proteins anchoring on the envelope membrane surface of the SARS-CoV particles.
The M protein is a typical transmembrane glycoprotein composed of a triple- membrane domain spanning 80 amino acids that account for about one-third of the entire protein (221 residues in total).
Acting as the most abundant structural protein in the virion of SARS-CoV, M also plays a significant role in virus-specific humoral response and is able to elicit efficient neutralizing antibodies in SARS patients.
The E protein is a small integral membrane polypeptide that forms an ion channel. Either the absence or inactivation of the E protein results in attenuated virulence because of the alterations in virion morphology or tropism.
…
Conclusion:
The M protein of SARS-CoV holds dominant cellular immunogenicity. This, together with previous reports of a strong humoral response against the M protein, may help to further explain the immunogenicity of SARS and serves as potential targets for SARS-CoV vaccine design.
We conclude that the SARS-CoV M protein acts as a dominant immunogen for both the humoral response (from previous reported data) and cellular immune response.
SARS-2 membrane (M) protein is highly immunogenic, eliciting antibodies likely neutralizing
Jörrißen et al. (2021) Antibody Response to SARS-CoV-2 Membrane Protein in Patients of the Acute and Convalescent Phase of COVID-19:
[O]ur results suggest that during the acute phase of COVID-19, antibodies are raised to two linear epitopes of the SARS-CoV-2 M protein, located at the very N- and C-termini, showing almost similar levels of reactivity as immunodominant linear epitopes derived from the spike and nucleocapsid protein.
Anti-M is also present in the convalescent phase of COVID-19 patients, however at lower levels, with the N-terminus of the M protein as a preferred target.
In silico prediction of B cell epitopes was employed for the structural proteins, including S and M proteins.
The M protein may also harbor B cell epitopes that can mount a neutralizing antibody response as suggested for SARS-CoV-1.
From broad mapping studies using antibody samples of COVID-19 patients, B cell epitopes located in the S [spike] and N [nucleocapsid] protein were shown to be immunodominant.
…
The immune response directed to the virus spike protein has raised much interest, since this protein directs major arms of the immune system, e.g., by induction of neutralizing antibodies.
However, limited information about the properties of the SARS CoV-2 membrane protein is available.
To our knowledge, a detailed characterization of immunodominant B-cell epitopes in the membrane protein of SARS CoV-2 during the acute and convalescent phase of COVID-19 is compared here for the first time.
As the M protein of coronaviruses represents the most abundant protein of the viral particle, a presumably high level of antibody reactivity can be expected during SARS-CoV-2 infection.
Our data suggest that the level of antibody response recognizing linear epitopes of the M protein is in the same range as for epitopes of other structural viral proteins, namely, the spike protein and the nucleocapsid protein, corroborating that the M protein is highly immunogenic in COVID-19 patients.
[O]ur data indicate that during the first weeks of COVID-19, an IgM- and IgG-specific antibody response is raised in a significant portion of COVID-19 patients to the N- and C-terminal ends of the M protein, with almost identical levels as observed for epitopes located in the S and N protein.
In our analyses covering COVID-19 patients from the acute phase, a significant portion of patients had IgM and IgG antibodies against peptide M01 before day 20 PIO, with the earliest detection at day 8 PIO [post-illness onset].
Of note, the IgM antibody reactivity directed against peptide M01 was found to be increased following day 20 PIO, suggesting that the N-terminus is increasingly stimulating an IgM B cell response during acute disease.
Peptide M21 was recognized by fewer patients in the acute phase suggesting that this epitope is less immunogenic.
Although reactivities of antibodies to peptides M01, M21, S and N were altogether lower in the convalescent phase of COVID-19, a portion of the patients contained antibodies against peptide M01 at this phase, suggesting that antibodies directed to the N-terminus of the M protein may persist in the convalescent phase, however at lower levels.
It was concluded that the level of antibodies to linear epitopes of SARS-CoV-2 proteins, including peptides S and N, correlates to the severity of the disease (21).
[A]ntibody reactivity against peptide M01 was in the same range as directed to immunodominant epitopes present in peptides S and N in both cohorts, suggesting that similar antibody responses to all SARS-CoV-2 structural proteins are mounted in the two phases of COVID-19.
Antibodies to several B cell epitopes of the spike protein were identified to induce neutralizing antibodies. RBD was characterized to harbor major epitopes of the neutralizing antibody response.
Based on mostly theoretical and in silico analyses, M protein of SARS-CoV-2 was also suggested to represent a candidate of vaccines.
Whether antibodies directed to the M protein can neutralize virus as suggested for SARS-CoV-1 is not known.





https://youtu.be/R000hqe2fIw
My long-held belief is because they want it to be the "SARS-CoV-2" S spike and nothing else, preferably coded by mRNA wrapped in LNP. It works as they want it to work, and they won't have it any other way.